Volunteers in U.K. Deliberately Infected with the Coronavirus for Vaccine Trials
October 26, 2020
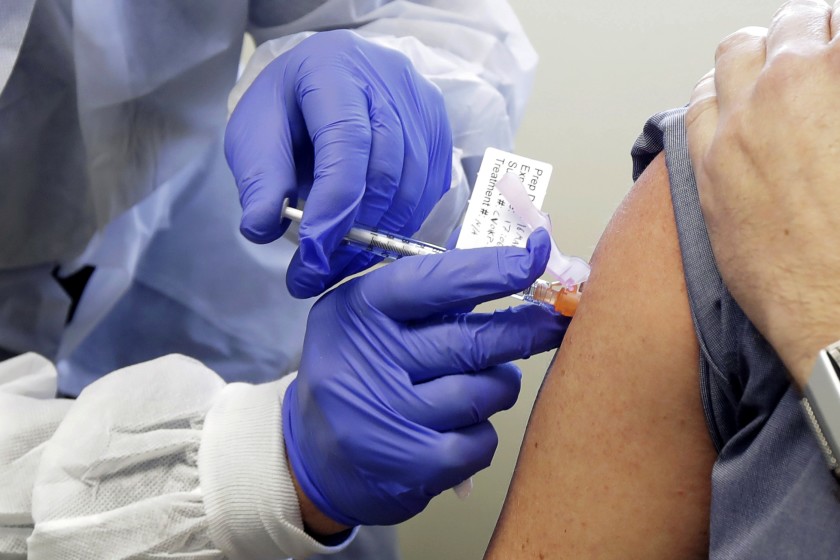
Researchers in the United Kingdom are asking young, healthy volunteers to deliberately infect themselves with the coronavirus, in order to study how people immunized with different vaccines respond to the virus. This past Tuesday, the U.K. government announced that it is spending 33.6 million pounds (roughly $43.5 million) in the first stage of a series of studies, known as human challenge trials. Human challenge trials are known to provide insight into diseases including malaria and influenza. The testing is scheduled to begin in January at a quarantine facility in London.
In stage one, the researchers will determine the smallest dosage required to reliably infect the volunteers, between the ages of 18 to 30. If that stage passes regulatory and ethical approval, the U.K. government mentioned the trials are estimated to take place next January to March. While supporters state challenge trials will lessen the time taken for the world to access an effective vaccine, critics argue that the potentially dangerous long-term effects of the virus are still unknown. “People are divided because it’s an ethical conundrum,” Sue Tansey, a pharmaceutical physician who is a member of the Nuffield Council on Bioethics, said. Despite rising moral questions, over 38,000 individuals have demonstrated an interest in volunteering for this cause.
If stage one is successfully executed, researchers will begin to immunize the group of coronavirus candidates, and then purposely infect them. Andrew Catchpole, a virologist and the chief scientific officer at Open Orphan, envisions that some trial participants will receive a placebo injection instead of a vaccine, or as an alternative experiment, comparative trials with two or more vaccines may take place. The vaccines for testing have not been announced thus far, as there are 150 vaccines in development around the globe, and some have even reached phase three tests.
Those participating in the challenge trials will be given the antiviral drug, remdesivir, which has been authorized for temporary use for Covid-19 in 50 countries. Experts in medical ethics, however, are in question of the trials because there is simply no highly effective treatment for the coronavirus. In fact, the World Health Organization recently reported the drug had no substantial effect on mortality when tested in clinical trials amongst 30 countries. Unfortunately, the worldwide death toll of the virus has exceeded 1.1 million people, thus “it is really vital that [researchers] move as fast as possible toward[s] getting effective vaccines and other treatments for Covid-19, “ Peter Openshaw, an immunologist at Imperial College London and a co-investigator on the study, said. Although the concept of purposely infecting volunteers with the virus surrounds uncharted medical territory, the potential results of the trials could dramatically speed up the time taken for people globally to access an efficient vaccine.